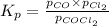Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, previously evacuated flask at 690 k, the pressure in the flask is initially 1.0 atm. after the reaction reaches equilibrium at 690 k, the total pressure in the flask is 1.2 atm. what is the value of kp for the reaction at 690 k?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, pr...
Questions

English, 13.11.2020 09:20


English, 13.11.2020 09:20


Social Studies, 13.11.2020 09:20


Mathematics, 13.11.2020 09:20


Chemistry, 13.11.2020 09:20


Social Studies, 13.11.2020 09:20

Mathematics, 13.11.2020 09:20

Mathematics, 13.11.2020 09:20

Mathematics, 13.11.2020 09:20


Biology, 13.11.2020 09:20


Advanced Placement (AP), 13.11.2020 09:20

Mathematics, 13.11.2020 09:20

Mathematics, 13.11.2020 09:20

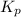 for the reaction at 690 K is 0.05
for the reaction at 690 K is 0.05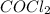 = 1.0 atm
= 1.0 atm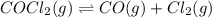
![[(1 - x) + x+ x]=1.2\\\\x=0.2atm](/tpl/images/0292/9648/696d1.png)