Chemistry, 06.10.2019 09:02 eastonstelter
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide from a solution containing rhodium(iii) sulfate according to the following balanced chemical equation: rh₂(so₄)₃(aq) + 6naoh(aq) → 2rh(oh)₃(s) + 3na₂so₄(aq) of 0.730 g of rhodium(iii) hydroxide is produced, what mass of sodium sulfate is also produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide...
Questions


English, 10.12.2020 06:40


Mathematics, 10.12.2020 06:40



Mathematics, 10.12.2020 06:40




English, 10.12.2020 06:40

World Languages, 10.12.2020 06:40






Biology, 10.12.2020 06:40

Mathematics, 10.12.2020 06:40

Mathematics, 10.12.2020 06:40

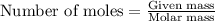 .....(1)
.....(1)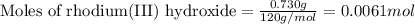

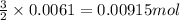 of sodium sulfate is also produced
of sodium sulfate is also produced