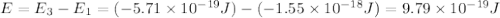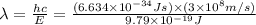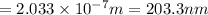Chemistry, 06.10.2019 05:30 kaylarae1930
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e2 −1.05 × 10−18 j e1 −1.55 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e...
Questions

Geography, 05.10.2019 19:00

Chemistry, 05.10.2019 19:00


History, 05.10.2019 19:00










Social Studies, 05.10.2019 19:00


Mathematics, 05.10.2019 19:00

Mathematics, 05.10.2019 19:00



Biology, 05.10.2019 19:00

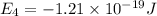

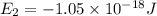
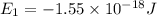
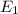 to
to  .
.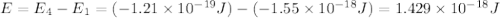
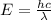
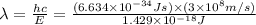
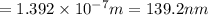
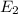 to
to 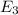 .
.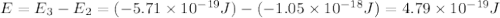
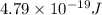 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from