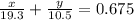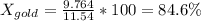Apiece of gold jewelry weighs 11.54 g and has a volume of 0.675 cm3. the jewelry contains only gold (density = 19.3 g/cm3) and silver (density = 10.5 g/cm3). assuming that the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold in the jewelry.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Apiece of gold jewelry weighs 11.54 g and has a volume of 0.675 cm3. the jewelry contains only gold...
Questions



Health, 20.07.2019 11:30


Mathematics, 20.07.2019 11:30




History, 20.07.2019 11:30


Mathematics, 20.07.2019 11:30


Mathematics, 20.07.2019 11:30