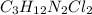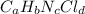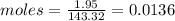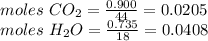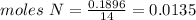Chemistry, 04.10.2019 20:20 estrellagutierrez12
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in water and allowed to react with excess silver nitrate, agno3, all the chlorine in x reacts and 1.95 g of solid agcl is formed. when 1.00 g of x undergoes complete combustion, 0.900 g of co2 and 0.735 g of h2o are formed. what is the empirical formula of x?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in...
Questions






Biology, 28.07.2019 17:30






Chemistry, 28.07.2019 17:30





Mathematics, 28.07.2019 17:30



Business, 28.07.2019 17:30