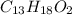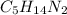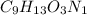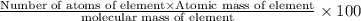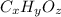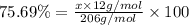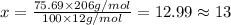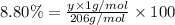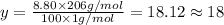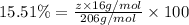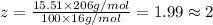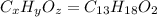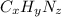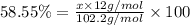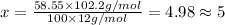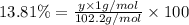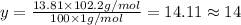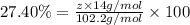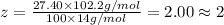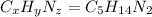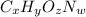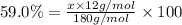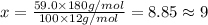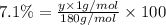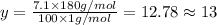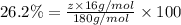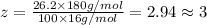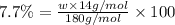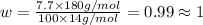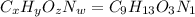Chemistry, 06.10.2019 02:30 xwalker6772
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass of 206 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part b cadaverine, a foul-smelling substance produced by the action of bacteria on meat, contains 58.55% c, 13.81% h, and 27.40% n by mass; its molar mass is 102.2 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part c epinephrine (adrenaline), a hormone secreted into the bloodstream in times of danger or stress, contains 59.0% c, 7.1% h, 26.2% o, and 7.7% n by mass; its molar mass is about 180 amu. express your answers as chemical formulas separated by a comma. nothing

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass...
Questions


History, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00




Mathematics, 24.07.2019 20:00


History, 24.07.2019 20:00


History, 24.07.2019 20:00






Social Studies, 24.07.2019 20:00