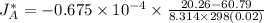Chemistry, 03.10.2019 05:00 genyjoannerubiera
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kpa pressure and 298 k. at one point, the partial pressure of methane is pa1 = 60.79 kpa, and at a point 0.02 m distance away, pa2 = 20.26 kpa. if the total pressure is constant throughout the tube, calculate the flux of ch4 (methane) at steady state for equimolar counterdiffusion.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
18.1-1. diffusion of methane through helium. a gas of ch4 and he is contained in a tube at 101.32 kp...
Questions



History, 29.08.2019 08:30

Chemistry, 29.08.2019 08:30




History, 29.08.2019 08:30

History, 29.08.2019 08:30



Mathematics, 29.08.2019 08:30





Mathematics, 29.08.2019 08:30


Biology, 29.08.2019 08:30

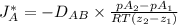
 = 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)
= 0.675 × 10⁻⁴ m²/s (for He-CH4 at 101.32 kPa and 298 K)