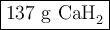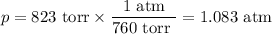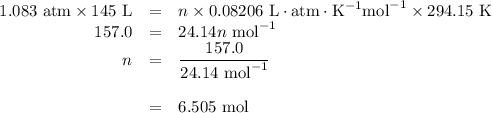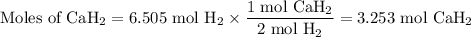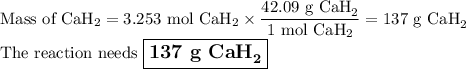Calcium hydride, cah2, reacts with water to form hydrogen gas:
cah2(s)+2h2o(l)→ca(oh)2(aq)+2...

Calcium hydride, cah2, reacts with water to form hydrogen gas:
cah2(s)+2h2o(l)→ca(oh)2(aq)+2h2(g)
this reaction is sometimes used to inflate life rafts, weather balloons, and the like, where a simple, compact means of generating h2 is desired.
how many grams of cah2 are needed to generate 145 l of h2 gas if the pressure of h2 is 823 torr at 21 ∘c?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Questions

History, 25.03.2021 18:30

History, 25.03.2021 18:30

Mathematics, 25.03.2021 18:30

Mathematics, 25.03.2021 18:30



History, 25.03.2021 18:30



History, 25.03.2021 18:30

Mathematics, 25.03.2021 18:30


Mathematics, 25.03.2021 18:30

Computers and Technology, 25.03.2021 18:30


Mathematics, 25.03.2021 18:30

English, 25.03.2021 18:30


Mathematics, 25.03.2021 18:30