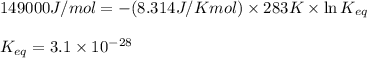Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
For a certain chemical reaction, the standard gibbs free energy of reaction at 10.0 °c is 149. kj. c...
Questions






Mathematics, 03.07.2019 18:00

Computers and Technology, 03.07.2019 18:00

Chemistry, 03.07.2019 18:00

Social Studies, 03.07.2019 18:00


English, 03.07.2019 18:00

Mathematics, 03.07.2019 18:00



History, 03.07.2019 18:00

Mathematics, 03.07.2019 18:00


Computers and Technology, 03.07.2019 18:00


History, 03.07.2019 18:00

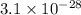
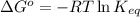
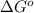 = standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
= standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )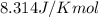
![15^oC=[273+15]K=283K](/tpl/images/0284/3257/82fe2.png)
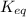 = equilibrium constant at 10°C = ?
= equilibrium constant at 10°C = ?