
Chemistry, 02.10.2019 21:30 Milanchik28
When 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter your question has been answered let us know if you got a answer. rate this answer question: when 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter at when 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50°c, the temperature rises to 30.17°c. calculate δh of this reaction. (assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as for pure water.) (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.) kj/mol h2o ?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
When 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter your qu...
Questions

Geography, 15.12.2019 03:31





Social Studies, 15.12.2019 03:31



English, 15.12.2019 03:31




History, 15.12.2019 03:31


Mathematics, 15.12.2019 03:31





English, 15.12.2019 03:31

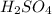 = 0.500 M
= 0.500 M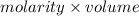
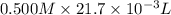
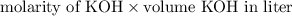
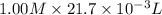
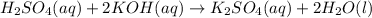
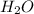 formed =
formed = 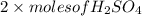 = (moles of KOH)
= (moles of KOH)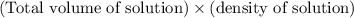
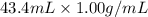
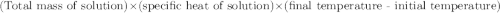
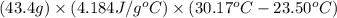
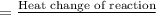
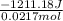
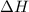 of the given reaction is -55.8 kJ/mol.
of the given reaction is -55.8 kJ/mol.

