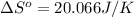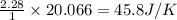Chemistry, 02.10.2019 21:30 dontworry48
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies at 298k, calculate the entropy change for the system when 2.28 moles of h2(g)react at standard conditions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1

Chemistry, 23.06.2019 15:30
Can someone me with these problems? see the attachment, .
Answers: 1

Chemistry, 23.06.2019 18:50
Why are very high temperatures and pressures required for fusion to occur? to generate the neutrons that are needed to break the nuclei o to overcome the repulsion between the protons in the nuclei that join to maintain the proper conditions to keep the chain reaction going to keep the uranium fuel separate from the control rods
Answers: 1
You know the right answer?
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies...
using standard absolute entropies...
Questions

Chemistry, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Biology, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

History, 10.09.2020 01:01

Chemistry, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Physics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

Business, 10.09.2020 01:01

English, 10.09.2020 01:01

English, 10.09.2020 01:01

Social Studies, 10.09.2020 01:01

English, 10.09.2020 01:01

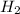 reacts at standard condition is 45.8 J/K
reacts at standard condition is 45.8 J/K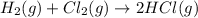
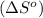 is:
is: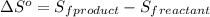
![\Delta S^o=[n_{HCl}\times \Delta S_f^0_{(HCl)}]-[n_{H_2}\times \Delta S_f^0_{(H_2)}+n_{Cl_2}\times \Delta S_f^0_{(Cl_2)}]](/tpl/images/0284/2286/d7d45.png)
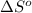 = entropy change of reaction = ?
= entropy change of reaction = ?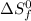 = standard entropy of formation
= standard entropy of formation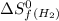 = 130.684 J/mol.K
= 130.684 J/mol.K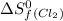 = 223.066 J/mol.K
= 223.066 J/mol.K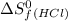 = 186.908 J/mol.K
= 186.908 J/mol.K![\Delta S^o=[2mole\times (186.908J/K.mole)]-[1mole\times (130.684J/K.mole)+1mole\times (223.066J/K.mole)}]](/tpl/images/0284/2286/55b6e.png)