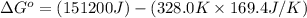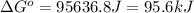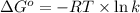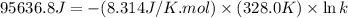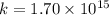Chemistry, 02.10.2019 21:30 bluebabyyy
For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4 j/k
the equilibrium constant for this reaction at 328.0 k is

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4...
h° = 151.2 kj and s° = 169.4...
Questions




Mathematics, 27.08.2019 13:30


Mathematics, 27.08.2019 13:30






Computers and Technology, 27.08.2019 13:30

Mathematics, 27.08.2019 13:30

Computers and Technology, 27.08.2019 13:30



World Languages, 27.08.2019 13:30



Computers and Technology, 27.08.2019 13:30


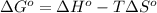
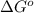 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?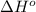 = standard enthalpy = 151.2 kJ = 151200 J
= standard enthalpy = 151.2 kJ = 151200 J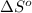 = standard entropy = 169.4 J/K
= standard entropy = 169.4 J/K