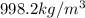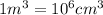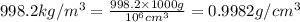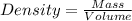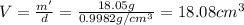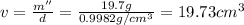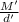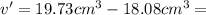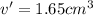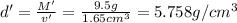Chemistry, 02.10.2019 21:30 timjape3g3z
Apycnometer is a precisely weighted vessel that is used for highly accurate density determinations. suppose that a pycnometer has a mass of 27.60 g when it is empty and has a mass of 45.65 g when it is completely filled with water at 20 °c. a 9.5 g metal ingot is placed in the pycnometer. when it is then filled with water at 20 °c, the total mass is 56.83 g. if the density of water at 20 °c is 998.2 kg/m3, what is the density of the metal ingot in grams per cubic centimeter.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Apycnometer is a precisely weighted vessel that is used for highly accurate density determinations....
Questions

Mathematics, 02.03.2021 17:40

Mathematics, 02.03.2021 17:40


English, 02.03.2021 17:40

Mathematics, 02.03.2021 17:40


Mathematics, 02.03.2021 17:40

Mathematics, 02.03.2021 17:40



Computers and Technology, 02.03.2021 17:40

Computers and Technology, 02.03.2021 17:40



English, 02.03.2021 17:40

Geography, 02.03.2021 17:40


Mathematics, 02.03.2021 17:40

Mathematics, 02.03.2021 17:40

Mathematics, 02.03.2021 17:40