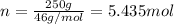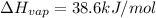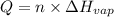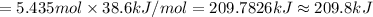Chemistry, 02.10.2019 22:00 adriandehoyos1p3hpwc
Ethanol, ch.0, is common beverage alcohol. at its boiling point of 78.5 °c, the enthalpy of vaporization of ethanol is 38.6 kj/mol. how much heat is required to vaporize 250 g of ethanol at 78.5 °c? ans = 209.8 kj

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

You know the right answer?
Ethanol, ch.0, is common beverage alcohol. at its boiling point of 78.5 °c, the enthalpy of vaporiza...
Questions

Physics, 28.10.2020 18:20




Computers and Technology, 28.10.2020 18:20


Mathematics, 28.10.2020 18:20

Social Studies, 28.10.2020 18:20

Mathematics, 28.10.2020 18:20



Social Studies, 28.10.2020 18:20


Computers and Technology, 28.10.2020 18:20


Mathematics, 28.10.2020 18:20


Mathematics, 28.10.2020 18:20