
Chemistry, 02.10.2019 22:00 TropicalFan
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in hz) and wavelength (in nm) of one of these photons.
frequency hz
and
wavelength nm
what is the total energy (in kj) in 1 mole of these photons?
kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Certain atoms emit photons of light with an energy of 3.820 ✕ 10−19 j. calculate the frequency (in h...
Questions


Mathematics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20


Chemistry, 01.03.2021 19:20

Chemistry, 01.03.2021 19:20



Geography, 01.03.2021 19:20



English, 01.03.2021 19:20


Social Studies, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20

English, 01.03.2021 19:20


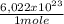 = 230040 J/mole ≡ 230,0 kJ/mole
= 230040 J/mole ≡ 230,0 kJ/mole

