
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and oils from becoming rancid.) a solution of 2.500 g of bht in 100.0 g of benzene had a freezing point of 4.880 oc. what is molecular mass of bht? . kf for benzene is 5.065 oc/m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and o...
Questions

Mathematics, 05.10.2019 16:40

Mathematics, 05.10.2019 16:40



Mathematics, 05.10.2019 16:40

Mathematics, 05.10.2019 16:40


Business, 05.10.2019 16:40

Biology, 05.10.2019 16:40

Chemistry, 05.10.2019 16:40

Mathematics, 05.10.2019 16:40


Biology, 05.10.2019 16:40


Computers and Technology, 05.10.2019 16:40

Mathematics, 05.10.2019 16:40

History, 05.10.2019 16:40



Mathematics, 05.10.2019 16:40

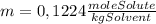
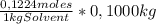 = 0,01224 moles of solute ≡ BHT
= 0,01224 moles of solute ≡ BHT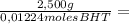 204,2 g/mol
204,2 g/mol

