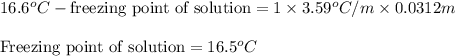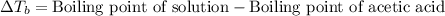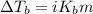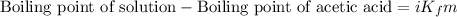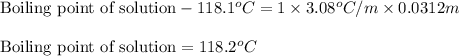Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Asolution was prepared by dissolving 0.800 g of sulfur s8, in 100.0 g of acetic acid, hc2h3o2. calcu...
Questions

Mathematics, 25.09.2021 22:00

English, 25.09.2021 22:00

Mathematics, 25.09.2021 22:00


Mathematics, 25.09.2021 22:00



English, 25.09.2021 22:00

English, 25.09.2021 22:00

Geography, 25.09.2021 22:00

Physics, 25.09.2021 22:00

History, 25.09.2021 22:00

Mathematics, 25.09.2021 22:00



Mathematics, 25.09.2021 22:00


Mathematics, 25.09.2021 22:00

Mathematics, 25.09.2021 22:00

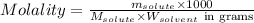
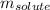 = Given mass of solute
= Given mass of solute 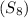 = 0.800 g
= 0.800 g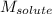 = Molar mass of solute
= Molar mass of solute  = 256.52 g/mol
= 256.52 g/mol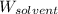 = Mass of solvent (acetic acid) = 100.0 g
= Mass of solvent (acetic acid) = 100.0 g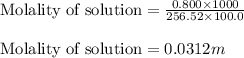
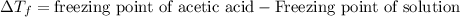
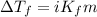
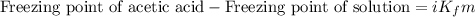
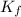 = molal freezing point depression constant = 3.59°C/m
= molal freezing point depression constant = 3.59°C/m