
Chemistry, 02.10.2019 22:00 hunterwilliams375
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benzene, c6h6? . the vapor pressure of pure benzene at 23 oc is 86.0 mm hg; the vapor pressure of naphthalene can be neglected. calculate the vapor-pressure lowering of the solution.

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benz...
Questions

Mathematics, 29.08.2021 23:50


Mathematics, 29.08.2021 23:50

Mathematics, 29.08.2021 23:50



Mathematics, 29.08.2021 23:50

English, 29.08.2021 23:50

Social Studies, 29.08.2021 23:50


Mathematics, 29.08.2021 23:50

Mathematics, 29.08.2021 23:50




Mathematics, 29.08.2021 23:50




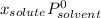 (1)
(1)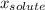 is molar fraction of solute
is molar fraction of solute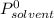 is the capor pressure of the pure solvent (86,0 mmHg)
is the capor pressure of the pure solvent (86,0 mmHg)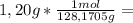 9,36x10⁻³ moles solute
9,36x10⁻³ moles solute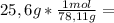 0,328 moles solvent
0,328 moles solvent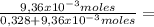 0,0278
0,0278

