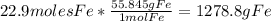Chemistry, 02.10.2019 21:20 fancycar14
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the balanced equation for this reaction? (use the lowest possible coefficients. omit states of matter.) b. what number of moles of iron reacts with 17.15 mol of oxygen from the air? mol c. what mass of iron is required to react with 17.15 mol of oxygen? mass =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the b...
Questions

English, 19.02.2021 21:30


Mathematics, 19.02.2021 21:30







Mathematics, 19.02.2021 21:30

Spanish, 19.02.2021 21:30


Social Studies, 19.02.2021 21:30



History, 19.02.2021 21:30

Mathematics, 19.02.2021 21:30

Spanish, 19.02.2021 21:30

Mathematics, 19.02.2021 21:30

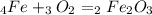
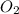
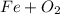
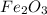 , so you should write the product:
, so you should write the product: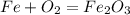
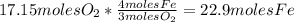
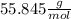 , so:
, so: