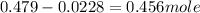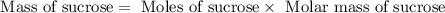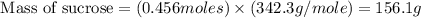The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order reaction with a rate constant of 1.8 x 10-45-1 at 25°c. determine the mass (g) of sucrose that is consumed when 2.15 l of a 0.223 m sucrose solution is allowed to react for 282 minutes. enter your answer as an integer. previous next not saved submit qu.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Aforce of attraction/repulsion due to the spin of electrons what is this force?
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order...
Questions

English, 12.12.2020 17:00

Chemistry, 12.12.2020 17:00


History, 12.12.2020 17:00

English, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Health, 12.12.2020 17:00


Biology, 12.12.2020 17:00



Mathematics, 12.12.2020 17:00

English, 12.12.2020 17:00

Advanced Placement (AP), 12.12.2020 17:00


Biology, 12.12.2020 17:00


Computers and Technology, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

![[C_t]=[C_o]e^{-kt}](/tpl/images/0284/1096/86a58.png)
![[C_t]](/tpl/images/0284/1096/81c01.png) = concentration of sucrose at time 't'
= concentration of sucrose at time 't'![[C_o]](/tpl/images/0284/1096/61f0a.png) = concentration of sucrose at time '0' = 0.223 M
= concentration of sucrose at time '0' = 0.223 M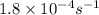
![[C_t]=(0.223)\times e^{-(1.8\times 10^{-4})\times (16920)}](/tpl/images/0284/1096/fccf3.png)
![[C_t]=0.0106M](/tpl/images/0284/1096/bb844.png)
![n_o=[C_o]\times V=0.223M\times 2.15L=0.479moles](/tpl/images/0284/1096/1c118.png)
![n_t=[C_t]\times V=0.0106M\times 2.15L=0.0228moles](/tpl/images/0284/1096/96015.png)