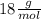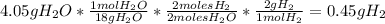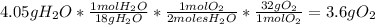Chemistry, 02.10.2019 21:00 MarMarMar07
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water is used.
2 h2o→2 h2+o2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water...
Questions



Chemistry, 27.03.2020 00:37



Mathematics, 27.03.2020 00:37



Mathematics, 27.03.2020 00:37

Biology, 27.03.2020 00:38



English, 27.03.2020 00:38

History, 27.03.2020 00:38






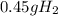
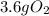
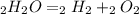
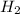 =
= 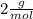
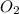 =
= 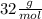
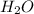 =
=