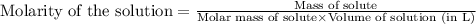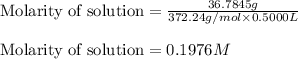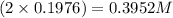Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 36.7845 grams of disodium acid dihydate in enough water to prep...
Questions



Mathematics, 22.05.2020 00:04



Biology, 22.05.2020 00:04

History, 22.05.2020 00:04

Mathematics, 22.05.2020 00:04

Physics, 22.05.2020 00:04

Computers and Technology, 22.05.2020 00:04



Geography, 22.05.2020 00:04



Biology, 22.05.2020 00:04