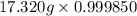Chemistry, 02.10.2019 21:00 11AnimalLover11
Liquid octane (ch) has a density of 0.7025 g/ml at 20 °c. find the true mass (murue) of octane when the mass weighed in 18 air is 17.320 g. assume the air density is 0.0012 g/ml and the balance weight density is 7.5 g/ml 17.35

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Liquid octane (ch) has a density of 0.7025 g/ml at 20 °c. find the true mass (murue) of octane when...
Questions


Mathematics, 10.02.2021 05:50

Mathematics, 10.02.2021 05:50

Chemistry, 10.02.2021 05:50

Spanish, 10.02.2021 05:50


Mathematics, 10.02.2021 05:50



Mathematics, 10.02.2021 05:50

Biology, 10.02.2021 05:50


English, 10.02.2021 05:50

Mathematics, 10.02.2021 05:50


Social Studies, 10.02.2021 05:50

Mathematics, 10.02.2021 05:50

English, 10.02.2021 05:50

Mathematics, 10.02.2021 05:50

Mathematics, 10.02.2021 05:50

![[m' \times \frac{1 - \frac{d_{a}}{d_{w}}}{1 - \frac{d_{a}}{d}}]](/tpl/images/0284/1391/dae17.png)
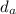 = density of air = 0.0012 g/ml
= density of air = 0.0012 g/ml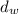 = density of the balance = 7.5 g/ml
= density of the balance = 7.5 g/ml![[17.320 g \times \frac{1 - \frac{0.0012 g/ml}{7.5 g/ml}}{1 - \frac{0.0012 g/ml}{0.7025}}]](/tpl/images/0284/1391/1b41f.png)