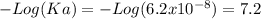Chemistry, 02.10.2019 20:20 keving4three
Aphosphate buffer is involved in the formation of urine. the developing urine contains h2po4 and hpo42- in the same concentration as present in blood plasma. identify the acid and its conjugate base. write the ionization equation and the ka expression. 6.2 x 10^(8) a. the ka of h2po4 is 6.2 x 108. what is the pka? is this acid weaker or stronger than h2co3? which buffer system is more optimal for regulating ph in the body? b. using lechatlier's principle, what happens if the urine is acidified (h+ ions added)?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
Aphosphate buffer is involved in the formation of urine. the developing urine contains h2po4 and hpo...
Questions


Mathematics, 03.10.2020 01:01





Mathematics, 03.10.2020 01:01

Chemistry, 03.10.2020 01:01

Mathematics, 03.10.2020 01:01

Mathematics, 03.10.2020 01:01



English, 03.10.2020 01:01



History, 03.10.2020 01:01


Mathematics, 03.10.2020 01:01


Mathematics, 03.10.2020 01:01

 ⇄
⇄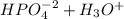 (1)
(1)![Ka = \frac{[HPO_{4}^{-2}] [H_{3}O^{+}]}{[H_{2}PO_{4}^{-}] [H_{2}O]} = 6.2x10^{-8}](/tpl/images/0284/0159/77441.png)