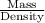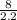A100.0 ml graduated cylinder is half-filled with 8.0 g of diatomaceous earth, a material consisting mostly of silica and used as a filtering medium in swimming pools. how many millilitres of water are required to fill the cylinder to the 100.0 ml mark? the diatomaceous earth is insoluble in water and has a density of 2.2 g/cm3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
A100.0 ml graduated cylinder is half-filled with 8.0 g of diatomaceous earth, a material consisting...
Questions

Mathematics, 21.08.2021 06:10

Mathematics, 21.08.2021 06:10

English, 21.08.2021 06:10

Mathematics, 21.08.2021 06:10

English, 21.08.2021 06:10

English, 21.08.2021 06:20

History, 21.08.2021 06:20


Mathematics, 21.08.2021 06:20


Mathematics, 21.08.2021 06:20

Mathematics, 21.08.2021 06:20


Physics, 21.08.2021 06:20