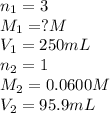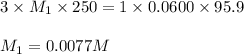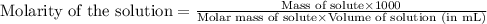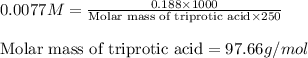Chemistry, 02.10.2019 02:00 Trevon0906
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask and dilutes to the mark with distilled water. he then titrates this solution with 0.0600 m naoh solutions. when the titration reaches the equivalence point, the chemist finds he has added 95.9 ml of naoh solution.
calculate the molar mass of the unknown acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask...
Questions

Chemistry, 04.11.2021 14:00

History, 04.11.2021 14:00






Mathematics, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Arts, 04.11.2021 14:00


Biology, 04.11.2021 14:00



Mathematics, 04.11.2021 14:00



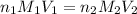
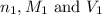 are the n-factor, molarity and volume of triprotic acid
are the n-factor, molarity and volume of triprotic acid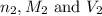 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.