
Chemistry, 01.10.2019 22:20 savannahckatz
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891 g . the two rings are heated to 66.887 oc and dropped into a 13.9 ml of water at 21.9 oc. when equilibrium is reached, the temperature of the water is 24.2 oc. what is the mass of gold ring? (assume a density of 0.998 g/ml for water.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3


Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Apure gold ring (c = 0.128 j/g°c) and pure silver ring (c = 0.235 j/g°c) have a total mass of 16.891...
Questions


Chemistry, 24.08.2019 00:30

Mathematics, 24.08.2019 00:30


Mathematics, 24.08.2019 00:30


Geography, 24.08.2019 00:30

History, 24.08.2019 00:30

History, 24.08.2019 00:30

Social Studies, 24.08.2019 00:30

Spanish, 24.08.2019 00:30



History, 24.08.2019 00:30

Business, 24.08.2019 00:30

English, 24.08.2019 00:30


English, 24.08.2019 00:30

Biology, 24.08.2019 00:30

Mathematics, 24.08.2019 00:30

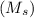 + mass of gold ring
+ mass of gold ring 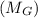
![[M_{G} \times 0.128 J/g^{o}C \times (66.887 - 24.2)^{o}C] + [M_{s} \times 0.235 J/g^{o}C \times (66.887 - 24.2)^{o}C]](/tpl/images/0281/0300/1110a.png) =
= 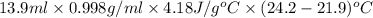
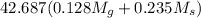 = 133.368
= 133.368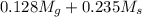 = 3.124
= 3.124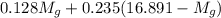 = 3.124
= 3.124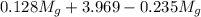 = 3.124
= 3.124 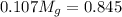
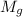 = 7.897 g
= 7.897 g

