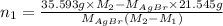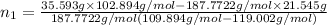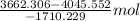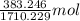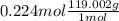Chemistry, 01.10.2019 22:00 GodlyGamer8239
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g mixture of kbr and nabr is dissolved in water, then a solution of agno3 is added so that all of the bromine present is converted to solid agbr. the agbr product is dried and found to have a mass of 35.593 g. what mass of kbr was present in the original mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
Both kbr and nabr are soluble ionic compounds and fully dissociate in aqueous solution. a 21.545-g m...
Questions

Chemistry, 09.12.2021 21:30



English, 09.12.2021 21:30

Mathematics, 09.12.2021 21:30


History, 09.12.2021 21:30


History, 09.12.2021 21:30

Chemistry, 09.12.2021 21:30


English, 09.12.2021 21:30


History, 09.12.2021 21:30


English, 09.12.2021 21:30

Mathematics, 09.12.2021 21:30

Computers and Technology, 09.12.2021 21:30


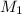 and NaBr is
and NaBr is 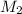 .
.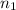 and
and 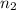 .
.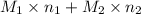 = 21.545 g
= 21.545 g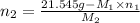
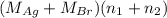 = 35.593 g
= 35.593 g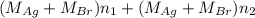 = 35.593 g
= 35.593 g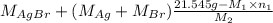 = 35.593 g
= 35.593 g