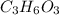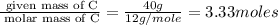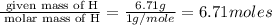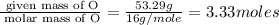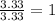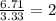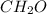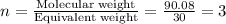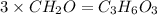Chemistry, 01.10.2019 17:30 MansellS5529
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in carbon, 6.71% mass in hydrogen, and the remaining mass in oxygen. determine its empirical formula. the formula mass of the unknown is independently determined to be 90.08 g/mol, determine the unknown’s molecular formula

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in car...
Questions

Mathematics, 30.11.2020 22:50


Computers and Technology, 30.11.2020 22:50



Advanced Placement (AP), 30.11.2020 22:50


Mathematics, 30.11.2020 22:50

History, 30.11.2020 22:50

Geography, 30.11.2020 22:50


Computers and Technology, 30.11.2020 22:50


Computers and Technology, 30.11.2020 22:50

English, 30.11.2020 22:50

Arts, 30.11.2020 22:50

English, 30.11.2020 22:50