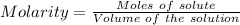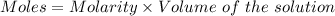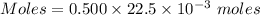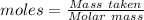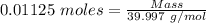Chemistry, 01.10.2019 17:30 christylam1606
Aquality control chemist at dow chemical tried to determine purity of naoh using titration. he measured out 0.500 g naoh sample and dissolved it in 20 ml water. 22.5 ml of a 0.500 mol/l hcl solution was used to reach the end point. assume the impurity did not react with hcl, what is the % purity of this naoh sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

You know the right answer?
Aquality control chemist at dow chemical tried to determine purity of naoh using titration. he measu...
Questions


Chemistry, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31



Mathematics, 22.01.2020 08:31



Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

History, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

History, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31