
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a capacity of 300.0 l and is filled with 35.0 atm of helium gas. room a has a capacity of 200.0 l and holds 20.0 atm of nitrogen gas. room b has 40.0 atm of oxygen gas in 180.0 l. a. if you open both rooms to the hallway, what would be pressure of each gas in the combined space of the hallway and two rooms? b. what would be the mol fraction of oxygen in that gas? c. after mixing the gases, if you let gas escape until the total pressure in the hallway was 1.2 atm, what would be the final partial pressure of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a cap...
Questions


Health, 06.07.2019 14:00

Health, 06.07.2019 14:00


History, 06.07.2019 14:00





Spanish, 06.07.2019 14:00

Mathematics, 06.07.2019 14:00

History, 06.07.2019 14:00


Computers and Technology, 06.07.2019 14:00

English, 06.07.2019 14:00


Health, 06.07.2019 14:00

History, 06.07.2019 14:00

Mathematics, 06.07.2019 14:00

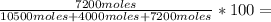 33,2% O₂
33,2% O₂

