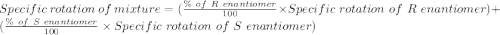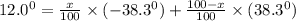Chemistry, 01.10.2019 04:10 mayahgrimes
An attempt at synthesizing a certain optically active compound resulted in a mixture of its enantiomers. the mixture had an observed specific rotation of 12.0°. if it is known that the specific rotation of the r enantiomer is –38.3°, determine the percentage of each isomer in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
An attempt at synthesizing a certain optically active compound resulted in a mixture of its enantiom...
Questions

Mathematics, 07.01.2020 22:31



Advanced Placement (AP), 07.01.2020 22:31


History, 07.01.2020 22:31




History, 07.01.2020 22:31

Advanced Placement (AP), 07.01.2020 22:31


Biology, 07.01.2020 22:31



Mathematics, 07.01.2020 22:31


Social Studies, 07.01.2020 22:31

History, 07.01.2020 22:31