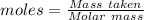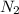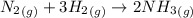Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions





Mathematics, 08.09.2021 08:50

Mathematics, 08.09.2021 08:50

Business, 08.09.2021 08:50

Mathematics, 08.09.2021 08:50

Mathematics, 08.09.2021 08:50


Mathematics, 08.09.2021 08:50



Mathematics, 08.09.2021 08:50


Social Studies, 08.09.2021 08:50

Computers and Technology, 08.09.2021 08:50

Mathematics, 08.09.2021 08:50

Mathematics, 08.09.2021 08:50

Computers and Technology, 08.09.2021 08:50

 .
.