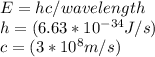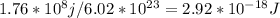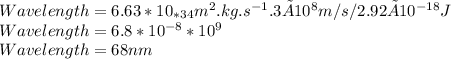Chemistry, 30.09.2019 18:20 mpgleboski
Determine the longest wavelength of light required to remove an electron from a sample of potassium metal, if the binding energy for an electron in k is 1,76x10*3 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
Determine the longest wavelength of light required to remove an electron from a sample of potassium...
Questions


Mathematics, 08.02.2021 20:20

Chemistry, 08.02.2021 20:20


English, 08.02.2021 20:20

Mathematics, 08.02.2021 20:20

History, 08.02.2021 20:20

History, 08.02.2021 20:20

Mathematics, 08.02.2021 20:20


Physics, 08.02.2021 20:20

Physics, 08.02.2021 20:20

Business, 08.02.2021 20:20

English, 08.02.2021 20:20

Mathematics, 08.02.2021 20:20

Mathematics, 08.02.2021 20:20



Mathematics, 08.02.2021 20:20

History, 08.02.2021 20:20