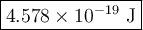Chemistry, 28.09.2019 23:10 zacksoccer8279
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission spectrum has a violet, a blue, a teal, and a red line. calculate the energy of one photon of this light.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission sp...
Questions





History, 12.01.2020 01:31

History, 12.01.2020 01:31


Computers and Technology, 12.01.2020 01:31


Advanced Placement (AP), 12.01.2020 01:31

Chemistry, 12.01.2020 01:31




English, 12.01.2020 01:31




Computers and Technology, 12.01.2020 01:31

Mathematics, 12.01.2020 01:31