
Chemistry, 28.09.2019 05:30 negritoboy014
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tumsr is 40.0 percent caco₃ by mass. using only tumsr, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions



History, 05.11.2021 04:50

English, 05.11.2021 04:50


English, 05.11.2021 04:50

Mathematics, 05.11.2021 04:50



Mathematics, 05.11.2021 04:50


English, 05.11.2021 04:50


Mathematics, 05.11.2021 04:50

Mathematics, 05.11.2021 05:00


Mathematics, 05.11.2021 05:10



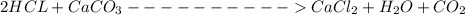
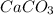 and HCl is 1 : 2
and HCl is 1 : 2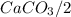 mol HCl = 0.1 mol
mol HCl = 0.1 mol 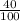 = 0.4
= 0.4 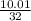 = 0.3128125 (number of containers)
= 0.3128125 (number of containers)


