
Chemistry, 28.09.2019 04:10 zacksoccer6937
Be sure to answer all parts. caffeine occurs naturally in coffee and tea, and is present in many soft drinks. the formula of caffeine is c, h1n402. calculate the formula mass and molar mass of caffeine. formula mass = amu molar mass = g/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

You know the right answer?
Be sure to answer all parts. caffeine occurs naturally in coffee and tea, and is present in many sof...
Questions


Mathematics, 23.01.2021 01:00

Geography, 23.01.2021 01:00

Geography, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

History, 23.01.2021 01:00


Mathematics, 23.01.2021 01:00


Mathematics, 23.01.2021 01:00


Biology, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00



Mathematics, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

Spanish, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00

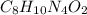
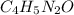
![(4\times 12)+(5\times 1)+(2\times 14)+(1\times 16)]=97amu](/tpl/images/0270/0693/7f107.png)
![(8\times 12)+(10\times 1)+(4\times 14)+(2\times 16)]=194g/mol](/tpl/images/0270/0693/8f300.png)


