
Chemistry, 28.09.2019 04:10 StephenCurry34
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k= 6.90 °c-kg-mol. calculate the freezing point of a solution made of 7.57g of sodium chloride (nacl) dissolved in 350. g of x round your answer to 3 significant digits. lºc x 5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

You know the right answer?
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k=...
Questions


Mathematics, 14.07.2020 22:01











Mathematics, 14.07.2020 22:01

Law, 14.07.2020 22:01


Mathematics, 14.07.2020 22:01


English, 14.07.2020 22:01


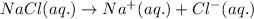
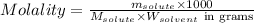
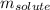 = Given mass of solute (NaCl) = 7.57 g
= Given mass of solute (NaCl) = 7.57 g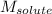 = Molar mass of solute (NaCl) = 58.44 g/mol
= Molar mass of solute (NaCl) = 58.44 g/mol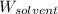 = Mass of solvent (liquid X) = 350.0 g
= Mass of solvent (liquid X) = 350.0 g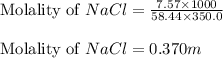
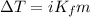
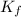 = molal freezing point depression constant = 6.90°C/m
= molal freezing point depression constant = 6.90°C/m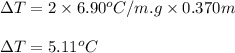
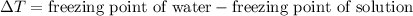
 = 5.11 °C
= 5.11 °C


