Chemistry, 28.09.2019 03:30 kyramks421
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alkane. termed hydrogenation, this type of reaction is used to produce products such as margarine. a typical hydrogenation reaction is c10h20() + h2(g) → c10h22(5) decene decane how much decane can be produced in a reaction of excess decene with 2.45 g hydrogen? give your answer in scientific notation. o *10 g decane

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1
You know the right answer?
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alk...
Questions


History, 02.01.2021 23:10

Health, 02.01.2021 23:10




Health, 02.01.2021 23:20


Mathematics, 02.01.2021 23:20




Biology, 02.01.2021 23:20


Chemistry, 02.01.2021 23:20





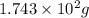
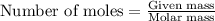 ......(1)
......(1)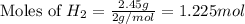
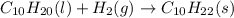
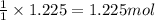 of decane
of decane