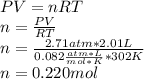Chemistry, 28.09.2019 03:30 hsjsjsjdjjd
Calculate the number of mol of helium in a 2.01-l balloon at 29°c and 2.71 atm of pressure. be sure to answer all parts. imol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Calculate the number of mol of helium in a 2.01-l balloon at 29°c and 2.71 atm of pressure. be sure...
Questions




English, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

Biology, 04.06.2021 18:30

Physics, 04.06.2021 18:30


Mathematics, 04.06.2021 18:30

Mathematics, 04.06.2021 18:30

English, 04.06.2021 18:30


Mathematics, 04.06.2021 18:30





History, 04.06.2021 18:30