
Chemistry, 28.09.2019 03:10 Tonyang1742
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g), respectively, is 191.5, 205.0, & 240.5 j/mol-k -156.0 j/k 156.0 j/k 120.5 j/k -120.5 j/k o ooo

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

You know the right answer?
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g...
Questions

Mathematics, 03.12.2019 13:31

History, 03.12.2019 13:31

English, 03.12.2019 13:31

Mathematics, 03.12.2019 13:31

Biology, 03.12.2019 13:31

History, 03.12.2019 13:31

Chemistry, 03.12.2019 13:31


English, 03.12.2019 13:31

Social Studies, 03.12.2019 13:31



Mathematics, 03.12.2019 13:31





History, 03.12.2019 13:31


History, 03.12.2019 13:31

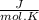 - [ 1 mol × 191,5
- [ 1 mol × 191,5 


