
Chemistry, 28.09.2019 02:30 ayoismeisjuam
Consider the gas reaction: h2 (g)2(g) 2hi (g) the equilibrium constant at 731 k is 50.3. equal amounts of all three gases (0.100 m) are introduced in a container, calculate the concentration of each gas after the system reaches equilibrium. express your results with the right number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1


Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
Consider the gas reaction: h2 (g)2(g) 2hi (g) the equilibrium constant at 731 k is 50.3. equal amou...
Questions

Biology, 10.11.2021 20:10





Mathematics, 10.11.2021 20:10





Physics, 10.11.2021 20:10




Mathematics, 10.11.2021 20:10





Mathematics, 10.11.2021 20:10

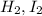 and
and 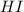 at equilibrium 0.033 M, 0.033 M and 0.234 M respectively.
at equilibrium 0.033 M, 0.033 M and 0.234 M respectively.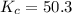
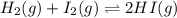
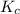 will be,
will be,![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0269/8551/8a740.png)
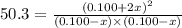
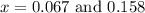
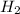 and
and 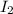 at equilibrium = (0.100-x) = 0.100 - 0.067 = 0.033 M
at equilibrium = (0.100-x) = 0.100 - 0.067 = 0.033 M

