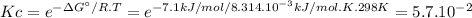Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Ag' for the isomerization reaction glucose-1-phosphate (gip) $ glucose-6-phosphate (g6p) is -7.1 kj/...
Questions

Biology, 17.10.2019 07:50




Physics, 17.10.2019 07:50

English, 17.10.2019 07:50


Mathematics, 17.10.2019 07:50





Business, 17.10.2019 07:50

History, 17.10.2019 07:50




History, 17.10.2019 07:50

Social Studies, 17.10.2019 07:50

Mathematics, 17.10.2019 07:50

![Kc=\frac{[G1P]}{[G6P]}](/tpl/images/0269/8214/83bf5.png)