
Chemistry, 28.09.2019 02:20 joejoefofana
Ideal he gas expanded at constant pressure of 3 atm until its volume was increased from 9 liters to 15 liters. during this process, the gas absorbed 800j of heat from the surroundings. calculate the internal energy change of the gas, ae.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Ideal he gas expanded at constant pressure of 3 atm until its volume was increased from 9 liters to...
Questions

Mathematics, 20.10.2020 02:01




Physics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

Arts, 20.10.2020 02:01


English, 20.10.2020 02:01


Computers and Technology, 20.10.2020 02:01

Medicine, 20.10.2020 02:01

History, 20.10.2020 02:01




Chemistry, 20.10.2020 02:01


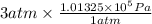

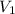 = 9 L =
= 9 L = 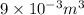 (as 1 L = 0.001
(as 1 L = 0.001 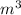 ),
),  = 15 L =
= 15 L = 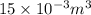
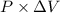
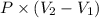

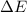 is as follows.
is as follows.

