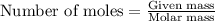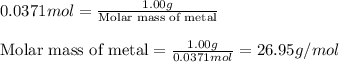Chemistry, 28.09.2019 02:10 sierranicole114
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.5000 m sulfuric acid. after all the metal had reacted, the remaining acid required 0.03340 l of 0.5000 m naoh solution for complete neutralization. calculate the molar mass of the metal and identify the element.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

You know the right answer?
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.50...
Questions

English, 18.03.2021 02:50

History, 18.03.2021 02:50




Mathematics, 18.03.2021 02:50

Biology, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50



Arts, 18.03.2021 02:50

Physics, 18.03.2021 02:50


History, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

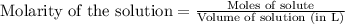 .....(1)
.....(1)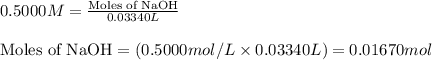

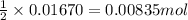 of sulfuric acid
of sulfuric acid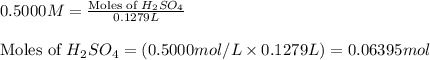
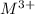 ion) and sulfuric acid follows:
ion) and sulfuric acid follows: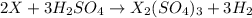
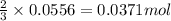 of metal
of metal