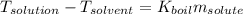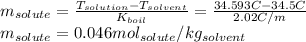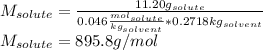The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) = 2.02 °c/m
in a laboratory experiment, students synthesized a new compound and found that when 11.20 grams of the compound were dissolved in 271.8 grams ofdiethyl ether, the solution began to boil at 34.593 °c. the compound was also found to be nonvolatile and a non-electrolyte.
what is the molecular weight they determined for this compound ?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) =...
Questions



Mathematics, 01.09.2019 09:50

Mathematics, 01.09.2019 09:50

Biology, 01.09.2019 09:50

Advanced Placement (AP), 01.09.2019 09:50


Mathematics, 01.09.2019 09:50

Mathematics, 01.09.2019 09:50




Social Studies, 01.09.2019 09:50

Chemistry, 01.09.2019 09:50

Chemistry, 01.09.2019 09:50

Social Studies, 01.09.2019 09:50


History, 01.09.2019 09:50