Chemistry, 28.09.2019 01:30 daltonrebekah3532
Propane can be turned into hydrogen by the two-step reforming process. in the first step, propane and water react to form carbon monoxide and hydrogen: c3hg(g)+3 3 co(g)+7 h2(g) in the second step, carbon monoxide and water react to form hydrogen and carbon dioxide: co(g)+h20(g)h2(9)+co2(g) write the net chemical equation for the production of hydrogen from propane and water. be sure your equation is balanced.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 18:00
If cos x =sin(20 + x) and 0 < x < 90, the value of x is > 3 , 35 , 350answer is 35, free 50 points
Answers: 1

You know the right answer?
Propane can be turned into hydrogen by the two-step reforming process. in the first step, propane an...
Questions

Chemistry, 08.04.2020 15:58


Social Studies, 08.04.2020 15:58





Mathematics, 08.04.2020 15:58

Health, 08.04.2020 15:58



Chemistry, 08.04.2020 15:58





World Languages, 08.04.2020 15:59


Computers and Technology, 08.04.2020 15:59

Geography, 08.04.2020 15:59

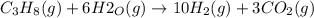
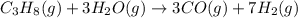 ...[1]
...[1]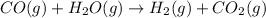 ...[2]
...[2]