
Chemistry, 28.09.2019 01:30 giavanleer14
Avoltaic electrochemical cell consists of a copper electrode in a cu2so4(aq) solution, and a palladium electrode in a pdso4(aq) solution at 25°c. the salt bridge consists of a solution of kcl(aq).
what is the concentration of the cu+if the concentration of the pdso4 is 0.498 m and the measured cell potential is 0.447 v?
given: cu+(aq) + e- ↔ cu(s) e°=+0.521 v
and pd2+(aq) + 2e- ↔ pd(s) e°=+0.987 v

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
Avoltaic electrochemical cell consists of a copper electrode in a cu2so4(aq) solution, and a palladi...
Questions

Mathematics, 12.05.2021 21:50

Biology, 12.05.2021 21:50

Mathematics, 12.05.2021 21:50


Biology, 12.05.2021 21:50






Mathematics, 12.05.2021 21:50





Mathematics, 12.05.2021 21:50

Mathematics, 12.05.2021 21:50


Mathematics, 12.05.2021 21:50

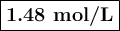
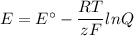
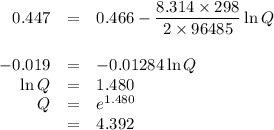
![\begin{array}{rcl}Q & = & \dfrac{\text{[Cu$^{+}$]}^{2}}{\text{[Pd]}}\\\\4.392 & = & \dfrac{{x}^{2}}{0.498}\\\\x^{2}& = & 2.187\\x & = & 1.48\\\end{array}\\\text{The concentration of Cu$^{+}$ is $\large \boxed{\textbf{1.48 mol/L}}$}](/tpl/images/0269/7404/9da92.png)


