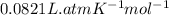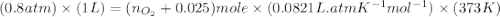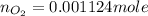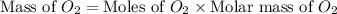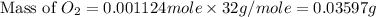Chemistry, 28.09.2019 01:20 bellaforlife9
Carbon dioxide (1.100g) was introduced into a 1l flask which contained some pure oxygen gas. the flask was warmed to 373k and the pressure was then found to be 608mmhg. if co2 and o2 were the only gases present, what was the mass of the oxygen in the flask?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
Carbon dioxide (1.100g) was introduced into a 1l flask which contained some pure oxygen gas. the fla...
Questions

Biology, 18.11.2020 21:50




History, 18.11.2020 21:50



Spanish, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50



History, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50

Chemistry, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50

Computers and Technology, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50

Biology, 18.11.2020 21:50

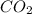 gas.
gas.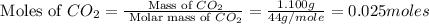
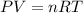
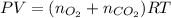
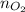 = number of moles of oxygen gas = ?
= number of moles of oxygen gas = ?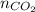 = number of moles of carbon dioxide gas = 0.025 mole
= number of moles of carbon dioxide gas = 0.025 mole