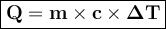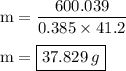Chemistry, 27.09.2019 17:30 Annaborden02
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a beaker containing 95.7 g of water at 22.7 ∘c. when the two substances reach thermal equilibrium, the final temperature is 24.2 ∘c. what is the mass of the copper block? express your answer in grams to three significant figures.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Ablock of copper of unknown mass has an initial temperature of 65.4 ∘c. the copper is immersed in a...
Questions


Business, 28.03.2021 19:20



Biology, 28.03.2021 19:20




Mathematics, 28.03.2021 19:20

Computers and Technology, 28.03.2021 19:20

English, 28.03.2021 19:20

English, 28.03.2021 19:20


English, 28.03.2021 19:20

Mathematics, 28.03.2021 19:20

English, 28.03.2021 19:20

Mathematics, 28.03.2021 19:20


Health, 28.03.2021 19:20